What is a Natural Product Number (NPN) and How to Get One in Canada?
- Vedic Ventures

- Aug 4, 2025
- 6 min read
Updated: Aug 18, 2025

If you’re planning to launch a supplement product in Canada, there’s one key regulatory requirement you cannot skip: the Natural Product Number (NPN). Whether you’re launching your own supplement brand or distributing existing formulas, understanding the NPN process is essential for compliance, credibility, and commercial success.
This number isn't just a regulatory checkbox, it's a badge of trust and quality.
In this in-depth guide, we’ll walk you through everything you need to know about NPNs: what they are, why they matter, and how to get one for your product.
What is a Natural Product Number (NPN)?
An NPN, or Natural Product Number, is an eight-digit number issued by Health Canada that appears on the label of licensed natural health products.
It signifies a natural health product (NHP) has been reviewed and approved for safety, efficacy, and quality.
Once a product is granted an NPN, it ensures your product meets all Canadian regulatory standards for sale to consumers and authorizes you to sell it in the Canadian market.
What Types of Products Require an NPN?
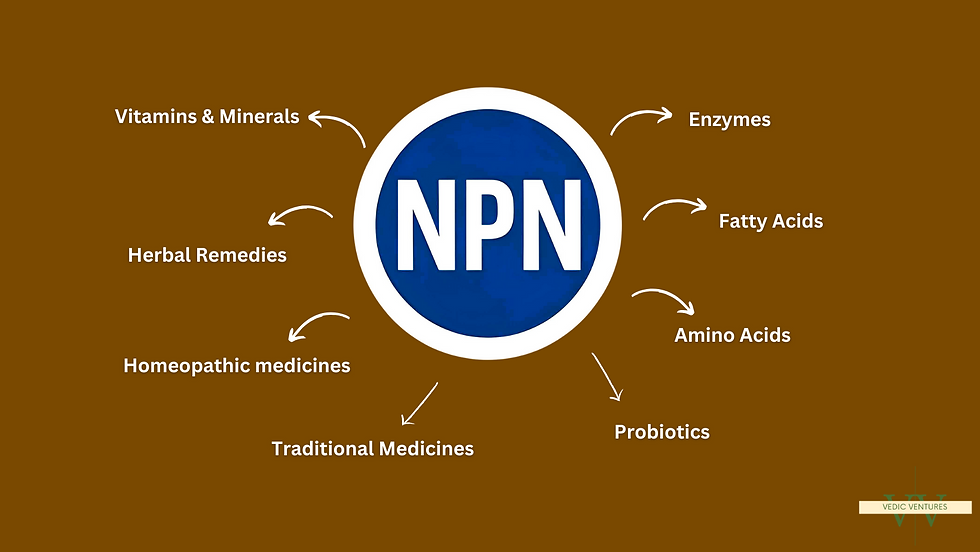
This system helps consumers make informed choices and ensures that only compliant products make it to store shelves or e-commerce platforms. An NPN is required for a wide range of products including:
Vitamins and minerals
Herbal remedies
Homeopathic medicines
Traditional medicines (e.g., Traditional Chinese Medicine, Ayurvedic medicine)
Probiotics
Amino acids and essential fatty acids
Enzymes
If your product makes a health claim or provides a physiological benefit, it will likely need an NPN before it can be legally sold in Canada.
Why Is Getting An NPN Important?

The Canadian market is tightly regulated, especially when it comes to health products.
Here’s why it matters:
Legal Compliance:
Selling supplements without an NPN in Canada is illegal. Non-compliance may result in product seizures, fines, or recall notices.
Market Access:
Major retailers and online platforms (including Amazon Canada) require NPNs to list health products.
Brand Reputation:
Having NPN-licensed products enhances your brand’s credibility and professionalism.
Builds Trust:
Having an NPN reassures both retailers and consumers that your supplement is safe and government-approved.
Enables Export :
International distributors value Health Canada’s stringent standards. An NPN enhances your brand’s export credibility.
For many supplement brands entering the Canadian market, obtaining an NPN is a gateway to long-term credibility and growth.
Who Issues the NPN and What’s the Governing Body?
NPNs are issued by the Natural and Non-Prescription Health Products Directorate (NNHPD), a branch of Health Canada. This body is responsible for evaluating the safety, efficacy, and quality of natural health products before they are made available to the public.
You can learn more directly from Health Canada's official page: Health Canada - Natural Health Products
Types of Product Licenses
Health Canada categorizes applications into three classes based on risk and ingredient complexity:
Class I: Straightforward products that use pre-approved ingredients with pre-cleared health claims.
Class II: Products that use a combination of approved and partially approved claims or ingredients.
Class III: High-risk or novel products with limited or no precedent in Health Canada's database.
Knowing your product’s classification helps set expectations around the timeline and documentation requirements.
How to Get an NPN in Canada
The NPN process can seem complex, but with the right steps, it’s entirely manageable, especially with expert guidance.
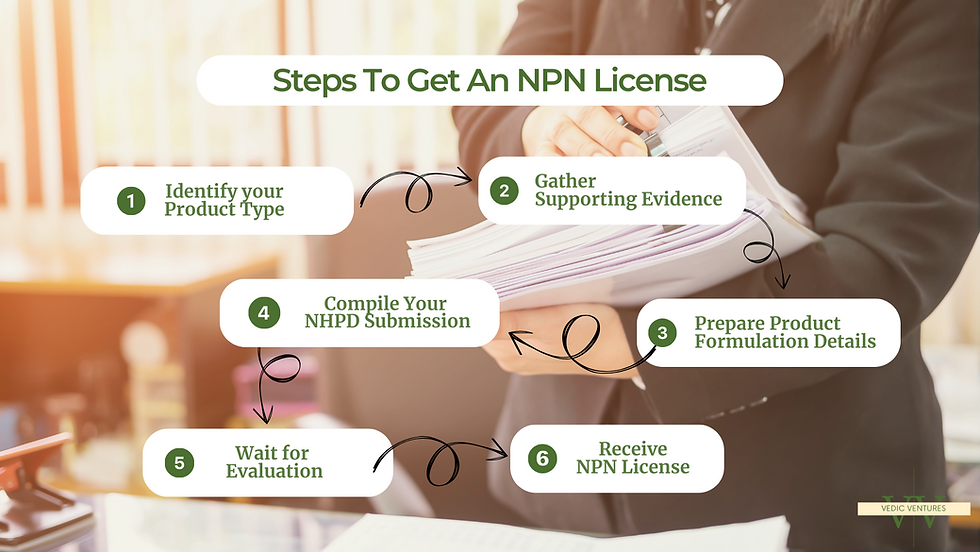
Step 1: Identify Your Product Type
Determine whether your product falls under the NHP category. Visit Health Canada’s official NHP Ingredients Database to confirm permitted ingredients.
Step 2: Gather Supporting Evidence
You must submit scientific evidence supporting the safety, efficacy, and dosage of your product. This may include:
Published research
Clinical Trials
Monographs from Health Canada
Historical usage data (for traditional products)
Step 3: Prepare Product Formulation Details
You’ll need to provide a detailed list of ingredients, their concentrations, dosage form, recommended use, warnings, and storage conditions.
For A Hassle Free NPN Submission - Contact Us Today
Step 4: Compile Your NHPD Submission
The application package includes:
NHP Application Form
Finished Product Specification Form
Evidence Summary Report
Label text or artwork
You can submit your application through the ePLA system (Electronic Product Licence Application).
Step 5: Wait for Evaluation
Health Canada will review the submission. This can take 60 to 210 days depending on the application class:
Class I: Uses existing monographs only
Class II: Uses partial monographs or some evidence
Class III: Requires full scientific review
Health Canada may request additional documentation during this process.
Tip: Work with a regulatory partner like Vedic Ventures where our experts bring over 25 years of experience to avoid mistakes and accelerate your approval.
Step 6: Receive NPN License
Once approved, you’ll be issued an 8 digit NPN and can legally manufacture, distribute, and market your product in Canada.
Who Can Apply for an NPN?
Any of the following may be the product license holder:
Brand owner
Canadian distributor
Private label company
However, only a company with a Canadian business number can hold the license. If you’re based outside Canada, you’ll need a Canadian representative.
Do I Also Need a Site License?
Any company that sells health supplement products in Canada must manufacture at a GMP licensed site. This license ensures the facility meets Good Manufacturing Practices (GMP) and maintains the highest quality standards.
But here’s the good news: you don’t need to own your own facility to bring your supplement brand to market!
Instead, you can partner with Vedic Ventures. This allows you to legally manufacture, package, and label your product while staying fully compliant without the cost and complexity of maintaining your own facility.
At Vedic Ventures, we handle all manufacturing and regulatory requirements under our affiliates site license. With over 25 years of expertise, our partner state-of-the-art affiliated facilities ensure your supplements are produced to GMP standards, helping you focus on building your brand while we take care of the rest.
Common Mistakes to Avoid During NPN Application
Submitting incomplete documentation
Using non-compliant ingredients or claims
Ignoring product classification
Poor-quality label designs
Misunderstanding monograph guidelines
Choosing the wrong application class
Not keeping track of new regulatory changes
What Happens After You Get the NPN?
Once approved:
You must print the NPN on the label.
You are responsible for post-market surveillance and product safety.
You must notify Health Canada of any changes in formulation or claims.
Keep records for audits.
Failure to comply after issuance can still result in enforcement action.
Need more guidance? Contact Us Today for a FREE Consultation
How Vedic Ventures Can Help
At Vedic Ventures, we assist brands in securing NPNs. We’ve helped dozens of supplement startups and health entrepreneurs navigate the Canadian regulatory process.
We help you:
Identifying ingredients and formulation strategy
Preparing your scientific dossier
Filing your ePLA application
Liaising with Health Canada on your behalf





